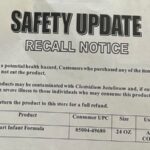
ByHeart has announced a nationwide recall of all its infant formula products, including cans and single-serve sticks, following an investigation into a multistate outbreak of infant botulism potentially linked to its “whole nutrition” infant formula, reports BritPanorama.
The US Food and Drug Administration (FDA) and the US Centers for Disease Control and Prevention (CDC) are currently investigating the outbreak, with preliminary epidemiological and laboratory data suggesting that ByHeart’s products may be contaminated with Clostridium botulinum, the bacterium responsible for botulism.
As of Monday, 15 children across 12 states, including Arizona, California, Illinois, and Texas, have been reported with suspected or confirmed cases of infant botulism linked to ByHeart formula. All affected infants, aged between 16 to 157 days, have been hospitalized and provided with BabyBIG, an intravenous antibody treatment, although no fatalities have been reported.
The FDA noted that since August, 84 infants have received treatment for infant botulism nationwide, with a significant proportion having been exposed to powdered infant formula, particularly ByHeart products. “This information shows that ByHeart brand formula is disproportionately represented among sick infants in this outbreak,” the FDA stated. Investigations are ongoing, although no other brands have been identified as posing risks.
In response to the outbreak, ByHeart co-founders Mia Funt and Ron Belldegrun have expressed their commitment to the investigation. They indicated that they are conducting extensive testing on all product batches and have granted the FDA comprehensive access to their facilities. “The most important thing for you to know is that all ByHeart product must be discarded. We know that switching formulas is not an easy process or decision,” they stated.
Preliminary lab results from California’s Department of Public Health indicated that the botulism-causing bacterium was detected in an open can of ByHeart formula fed to an infant with botulism. Despite this, the FDA confirmed that no unopened ByHeart products tested positive for Clostridium botulinum spores or toxins.
The fallout from this incident raises critical questions regarding safety protocols in the infant formula industry, especially as testing continues in several states. The monitoring of potential health risks remains a high priority as parents navigate this alarming situation.